1/4) New Research (Yesterday) in @Nature on the Memory of Your Fat Cells: “Adipose tissue retains an epigenetic memory of obesity after weight loss”
Let’s break it down…
You’re probably aware of the “yo-yo” effect, whereby people who lose excess weight are prone to gain it back.
But is this purely behavioral, or are there deeper metabolic mechanisms at play?
In this study, researchers took cell samples from human patients who were always lean versus those who had a history of obesity but who had lost weight after bariatric surgery, and measured gene expression profiles* from their fat at the time of surgery and 2 years later after substantial weight loss.
🧬They found significant changes in fat cells (adipocytes), as well as their precursors and also in other cell types, like the endothelial cells that line blood vessels.
Overall:
⚡️Fat cells from individuals with a history of obesity showed down-regulation (less expression of) genes relates to metabolic functions
🔥And up-regulation (more expression of) genes relates to inflammation functions
Thus, in the authors’ words, “These results indicate that obesity induces cellular and transcriptional (obesogenic) changes in the [fat cells], which are not resolved following significant weight loss."
Ref. Hinte et al. Nature Nov 18, 2024, doi: 10.1038/s41586-024-08165-7
Let’s break it down…
You’re probably aware of the “yo-yo” effect, whereby people who lose excess weight are prone to gain it back.
But is this purely behavioral, or are there deeper metabolic mechanisms at play?
In this study, researchers took cell samples from human patients who were always lean versus those who had a history of obesity but who had lost weight after bariatric surgery, and measured gene expression profiles* from their fat at the time of surgery and 2 years later after substantial weight loss.
🧬They found significant changes in fat cells (adipocytes), as well as their precursors and also in other cell types, like the endothelial cells that line blood vessels.
Overall:
⚡️Fat cells from individuals with a history of obesity showed down-regulation (less expression of) genes relates to metabolic functions
🔥And up-regulation (more expression of) genes relates to inflammation functions
Thus, in the authors’ words, “These results indicate that obesity induces cellular and transcriptional (obesogenic) changes in the [fat cells], which are not resolved following significant weight loss."
Ref. Hinte et al. Nature Nov 18, 2024, doi: 10.1038/s41586-024-08165-7
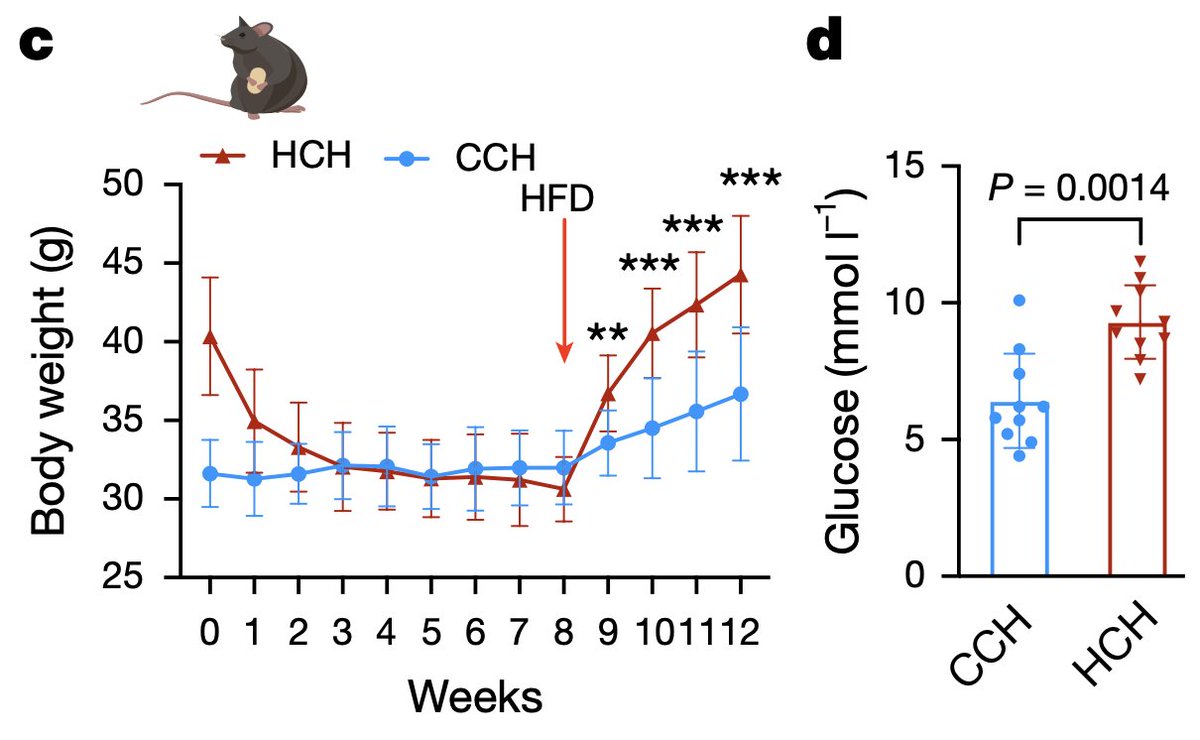
2/4) To get more granular, they did a similar experiment in mice where they fattened some mice using a high-sugar high-fat obesogenic diet, and then normalized their weight through dietary restriction and compared these to mice who never had obesity.
They found, consistent with the human data, persistently gene expression changes, including downregulation of metabolic pathways, such as fatty acid oxidation, mitochondrial signaling, etc., and upregulation of inflammatory pathways.
🔧How it works🔧
I’ll explain how this works at a high level through an analogy.
Your genetic code is like a book… even though all cells in your body contain your full genetic code they are different.
Why?
Because in different cells different pages are opened or shut. This determines the fat or function of cells.
What’s more, cells can “bookmark” or dogear pages for easy access. In the cell these are “epigenetic” changes, where tags are put on to DNA or the protein complexes around which DNA is wound.
This makes it easier (or harder) to access certain pages (certain genes), changing their expression profiles.
Hopefully that makes sense?
And that’s how cells develop a “memory” of past events, including the memory “I was once an ‘obese’ fat cell.” If it’s not too dark to say, think of it like PTSD for fat cells.
They found, consistent with the human data, persistently gene expression changes, including downregulation of metabolic pathways, such as fatty acid oxidation, mitochondrial signaling, etc., and upregulation of inflammatory pathways.
🔧How it works🔧
I’ll explain how this works at a high level through an analogy.
Your genetic code is like a book… even though all cells in your body contain your full genetic code they are different.
Why?
Because in different cells different pages are opened or shut. This determines the fat or function of cells.
What’s more, cells can “bookmark” or dogear pages for easy access. In the cell these are “epigenetic” changes, where tags are put on to DNA or the protein complexes around which DNA is wound.
This makes it easier (or harder) to access certain pages (certain genes), changing their expression profiles.
Hopefully that makes sense?
And that’s how cells develop a “memory” of past events, including the memory “I was once an ‘obese’ fat cell.” If it’s not too dark to say, think of it like PTSD for fat cells.
3/4) Now, are these changes functionally meaningful with respect to weight regain?
It would appear so. Human observational and clinical data suggest those who have lost weight are more prone to put weight back on.
Although, of course, in free living humans it’s hard to disentangle the effects of behavioral and constitutional (inborn) differences from those imposed from true epigenetic changes brought about by a history of obesity.
However, carefully controlled mouse experiments – which in this case should probably generalize to humans – do indeed strongly suggest that a history of obesity (red vs blue [control]) predisposes fat cells to take up sugar more readily, build up fat stores in response to insulin more quickly, and develop fatty liver more easily.
It would appear so. Human observational and clinical data suggest those who have lost weight are more prone to put weight back on.
Although, of course, in free living humans it’s hard to disentangle the effects of behavioral and constitutional (inborn) differences from those imposed from true epigenetic changes brought about by a history of obesity.
However, carefully controlled mouse experiments – which in this case should probably generalize to humans – do indeed strongly suggest that a history of obesity (red vs blue [control]) predisposes fat cells to take up sugar more readily, build up fat stores in response to insulin more quickly, and develop fatty liver more easily.
4/4) Those are the main findings from the paper I wanted to highlight. That said, some closing thoughts.
(1) It’s likely that a history of obesity – even if one is now lean – puts a person behind the eight-ball, metabolically speaking.
🤔But that doesn’t mean you’re doomed 🤔
Present lifestyle choices do have the dominant effect. And just because your road might be rockier, it doesn’t mean you shouldn’t or can’t traverse it.
Sorry. Biology is sometimes a jerk.
(2) The epigenetic memory is not specific to fat cells.
There were also changes, for example, in the endothelial cells that line blood vessels. Thus, a history of obesity could predispose individuals to diseases like cardiovascular disease cc @realDaveFeldman @AdrianSotoMota
Furthermore, there are many cell types that remain to be assessed for "obesity-induced epigenetic memory," like neurons and other brain cells @ChrisPalmerMD @hubermanlab @foundmyfitness
We’ve barely seen the tip of the iceberg with respect to how different past metabolic and environmental exposure impact gene expression across the body.
(3) 🧠Knowledge is Power! 🧠
Understanding these mechanisms is the first step to developing therapies/protocols to changes our epigenome and engineer our advantage over what nature seems to have planned.
Or, put another way, learning about mechanisms teaches us how to best take advantage of our evolutionary priming to work with, rather than against, nature’s (sometimes confusing) design.
(1) It’s likely that a history of obesity – even if one is now lean – puts a person behind the eight-ball, metabolically speaking.
🤔But that doesn’t mean you’re doomed 🤔
Present lifestyle choices do have the dominant effect. And just because your road might be rockier, it doesn’t mean you shouldn’t or can’t traverse it.
Sorry. Biology is sometimes a jerk.
(2) The epigenetic memory is not specific to fat cells.
There were also changes, for example, in the endothelial cells that line blood vessels. Thus, a history of obesity could predispose individuals to diseases like cardiovascular disease cc @realDaveFeldman @AdrianSotoMota
Furthermore, there are many cell types that remain to be assessed for "obesity-induced epigenetic memory," like neurons and other brain cells @ChrisPalmerMD @hubermanlab @foundmyfitness
We’ve barely seen the tip of the iceberg with respect to how different past metabolic and environmental exposure impact gene expression across the body.
(3) 🧠Knowledge is Power! 🧠
Understanding these mechanisms is the first step to developing therapies/protocols to changes our epigenome and engineer our advantage over what nature seems to have planned.
Or, put another way, learning about mechanisms teaches us how to best take advantage of our evolutionary priming to work with, rather than against, nature’s (sometimes confusing) design.
جاري تحميل الاقتراحات...